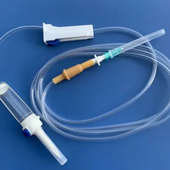
WHO-GMP, ISO 13485 Compliant Unit!
Fully Controlled Facility for Disposable Surgical Products Manufacturing!
Dispowell Surgicals is a CDSCO MDR, Government of India Approved plant with daily capacity of 1,00,000 set production. We have Inbuilt FULLY AUTOMATIC ETO Sterilization unit approved by Government for QMS having 1 Lakh+ Capacity per day. We have 0.3 Micron Clean assembly rooms to manufacture Non-pyrogenic products which is highest in segment. Plant is centralized AHU Enables which is very important for Export quality Products.
Dispowell Facility Includes:
Injection molding area
Extrusion Lines for tubing
0.3 Micron Clean Room for Assembly
Controlled Packaging Area
Leak Testing Facility
Inhouse ETO sterilization Unit
Automated Blister and Poly Packaging
Automated Pouch Sealing and Batch Printing
In-house Labs for Pyrogen, Sterility Test
Product Testing QA Labs
Fully Complaint Plant
SOP Operated Systems
Experienced Staff
We have Our Own Inbuilt Toxicity Testing Lab, Sterility Testing Lab and Quality Control/Assurance Labs to ensure top quality production Output. Our Labs are certified by NABL Accredited Agencies. We have our own Molds to manufacture spare parts of the finished good. This set-up help us to make the quality very consistent.
Chandrakant Patel
co founder

+
Manufacturing Facility
Supplying Worldwide!
Get the Quote!
Get instant quote of our Disposable surgical products via Email/Whatsapp, feel free to contact (+91) 9067680104.
Get QuoteCheck Full List of Our Products Click Here!